Evolution of Structure and Lithium Dynamics in LiNi0.8Mn0.1Co0.1O2 (NMC811) Cathodes during Electrochemical Cycling
Authors: Katharina Märker, Philip J. Reeves, Chao Xu, Kent J. Griffith, and Clare P. Grey
Case study author: Christopher O'Keefe
Department of Chemistry, University of Cambridge
The detailed information provided by NMR spectroscopy has made it an indispensable tool in the study of battery materials. Many of the cathode materials used in Li-ion batteries contain paramagnetic transition metal (TM) ions, which makes the acquisition of \(^7\)Li NMR spectra for these materials challenging due to rapidly decaying signals, broad features, and large hyperfine shifts. If the TMs are disordered in the cathode, these issues are exacerbated, and the assignment of the NMR spectra becomes more difficult. Expected hyperfine shifts of \(^7\)Li resonances due to interactions from individual TMs can be calculated using density functional theory (DFT); these contributions are (generally) additive such that the total \(^7\)Li hyperfine shift of each Li in the structure can be determined by considering the possible TMs in the first and second coordination sphere.
| 90° | 180° | |
|---|---|---|
| Ni\(^{2+}\) | –30 | 170 |
| Ni\(^{3+}\) | –15 | 110 |
| Mn\(^{4+}\) | 250 | –60 |
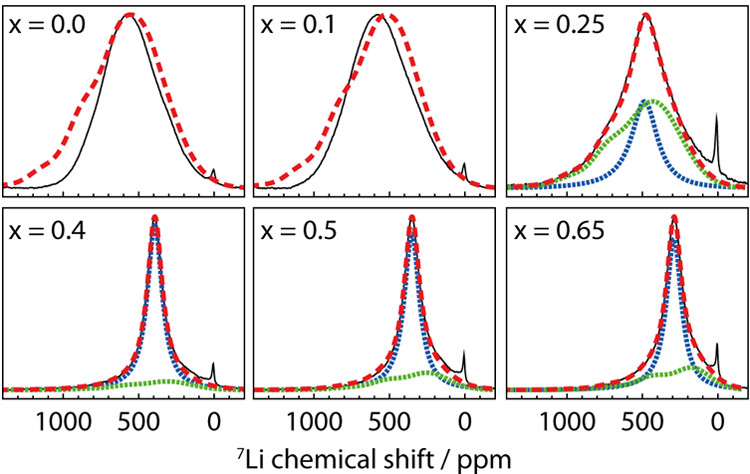
In a recent publication in Chem. Mater. 2019, 31, 2545-2554, this approach was used to understand the structural changes and Li dynamics in a high-capacity layered oxide cathode material, Li\(_{1-x}\)Ni\(_{0.8}\)Mn\(_{0.1}\)Co\(_{0.1}\)O\(_2\) (NMC811). Table 1 lists the expected hyperfine \(^7\)Li shifts from the paramagnetic TMs in the first (i.e., Li-O-TM bond angle of 90°) and second (i.e., Li-O-TM bond angle of 180°) coordination spheres. A \(^7\)Li spectrum (Figure 1) was then calculated for the pristine (i.e., x = 0) cathode material by considering the hyperfine shifts resulting from all possible 784 Li environments based on a random distribution of TMs. The calculated spectrum (red trace) matches reasonably well with the experimental spectrum (black trace), with discrepancies a result of possible TM ordering. At higher states of charge (i.e., 0.25 ≤ x ≤ 0.65), the experimental \(^7\)Li NMR patterns narrow, and spectra were calculated by considering changes in the bond pathway shifts (due to Ni oxidation and lattice expansion) and accounting for the effect of Li+ mobility. A combination of \(^7\)Li NMR and DFT was shown to be a useful tool to study the dynamics of Li ions in a challenging paramagnetic material. The characterization of Li mobility is crucial for understanding the rate performance of cathode materials. Our group has also developed bond pathway analysis to several other nuclei, including \(^{23}\)Na, \(^{31}\)P, \(^{25}\)Mg and \(^{17}\)O, enabling the evolution of the local structures of paramagnetic cathode materials to be closely monitored.